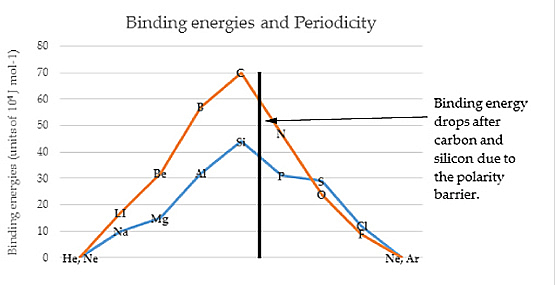
PART VSUB-ATOMIC PARTICLE PHYSICSCabibbo AngleA theoretical synthesis of data from a variety of sources in weak interaction processes generates a coupling angle. The magnitude of the angle came from accelerator particle collision by product measurements. This angle known as the Cabibbo angle has been measured from a variety of sources as 0.223 radian. In effect the sub-particles impact with a glancing impact. "The quarks with a definite mass, in this case the u, d, and s quarks are not the same as the quarks that couple to the weak mesons: they differ by a rotation in quark space through the Cabibbo angle. Why that should be so is something we do not really understand in a deep sense as yet" [1]. The theory of magnitude of angle stems from neutral current theory or quark theory. "The Cabibbo angle, is not determined by the electroweak theory (it is related to ratios of various Yukawa couplings) and is found experimentally to be about 13 degrees...the small ratio of the strangeness-changing to non-strangeness-changing charged current amplitudes is due to the smallness of the Cabibbo angle. It is worth emphasizing again that the standard model alone provides no understanding of the value of this angle" [2]. The Circular Model of the Atom provides insight as to the source of the Cabibbo angle. First, the drop in nuclear binding energy occurs when we measure the nucleons before the polarity barrier and those after the barrier. This evidences an energy differential which is central to the Circular Model.
Second, the analysis of shell level initial and terminal standing waves with positive and negative vector segments yield support for the new model. The electropositive segment of the electromagnetic wave is displaced (or leading) one wavelength forward in positive processes and in negative processes the displacement (or leading wavelength) is in the opposite direction by one wavelength. This positive and negative displacement carries the charge of the electron and positron respectively. With this displacement comes the clarification of the question of wave particle duality in photons. Current quantum theory has a discontinuity or successive bullet type energy theory of the photon in contrast to a classical wave. Any measurement, positive or negative would show a gap. However, on the electropositive vector process, it was bridged by the magnetic portion of the wave and in magnetic process, by electropositive segments of the electromagnetic wave. [1] Bernstein, J., 1989. The Tenth Dimension. New York: McGraw-Hill, p. 107. [2] Cooper, N. G. & West, G. B., Particle Physics: A Los Alamos Primer. Cambridge: Cambridge University Press, p. 71, emphasis added. |
 |
- Home
- The Circular Model
- Quantum Charts
- Model Physics
- Part I: supporting evidences
- introduction
- Pauli's exclusion principle
- dipole magnet
- unique electron flip
- polarity and anomalous angular momentum
- lanthanide contraction
- Stern-Gerlach
- electron tunneling
- discreteness
- electronegativity
- Compton effect
- Dirac's equation
- symmetry
- gyromagnetic ratio
- nulcear shells
- Kaluza-Klein
- gravity
- magnetism and monopoles
- Heisenburg uncertainty principle
- missing mass
- Olbers' paradox
- Big Bang
- Part II: spectral evidences
- Part III: fine structure constant
- Part IV: superconductivity
- Part V: sub-atomic particle physics
- Part VI: summary
- Part I: supporting evidences
- Astrophysics