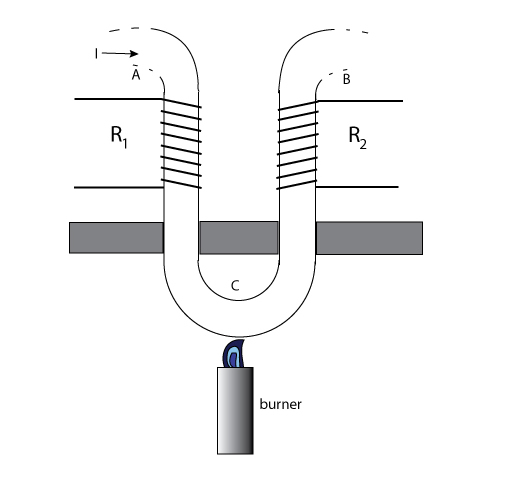
ADDENDUMTHOMSON EFFECT IN METALSThomson effect: "A phenomenon discovered in 1854 by William Thomson, later Lord Kelvin. He found that there occurs a reversible transverse heat flow into or out of a conductor of a particular metal, the direction depending upon whether a longitudinal electric current flows from colder to warmer metal or from warmer to colder. From these observations it may be shown that for copper there is a heat output where positive charge flows down a temperature gradient and a heat input where positive charge flows up a temperature gradient: whereas for iron the reverse is true. All metals may be divided into two classes with respect to the direction of the Thomson effect" [1]. A second scholar has noted and illustrated about the Thomson effect: "As in the preceding section, absorption of heat is to be taken as evidence for an emf that is acting in the same direction as that of the current, that is to say, electrical energy is being supplied at the expense of heat energy of the environment. Such is the case in the section AB. Likewise in the section AC, the current is opposed by an emf with consequent transformation of electrical energy into heat energy. Thus, in iron, the Thomson emf would give rise to a current in the iron from hot to cold regions. Many metals, including bismuth, cobalt, nickel and platinum, in addition to iron exhibit this same property, which is referred to as the negative Thomson effect. Another group of metals including antimony, cadmium, copper, and silver, display a positive Thomson effect; in these, the direction of the Thomson emf is such as to support a current within the metal from cold to hot regions. In one metal, lead, the Thomson effect is zero. In certain metals the effect reverses sign as the temperature is raised or as the crystal structure is altered" [2].
[1] Thomson Effect, 1983. McGraw-Hill Encyclopedia of Physics. New York: McGraw-Hill, p. 1175, emphasis added. [2] Duckworth, H. E., 1960. Electricity and Magnetism. Holt, Rinehart, and Wilson, p. 183, emphasis added.
|
 |
- Home
- The Circular Model
- Quantum Charts
- Model Physics
- Part I: supporting evidences
- introduction
- Pauli's exclusion principle
- dipole magnet
- unique electron flip
- polarity and anomalous angular momentum
- lanthanide contraction
- Stern-Gerlach
- electron tunneling
- discreteness
- electronegativity
- Compton effect
- Dirac's equation
- symmetry
- gyromagnetic ratio
- nulcear shells
- Kaluza-Klein
- gravity
- magnetism and monopoles
- Heisenburg uncertainty principle
- missing mass
- Olbers' paradox
- Big Bang
- Part II: spectral evidences
- Part III: fine structure constant
- Part IV: superconductivity
- Part V: sub-atomic particle physics
- Part VI: summary
- Part I: supporting evidences
- Astrophysics