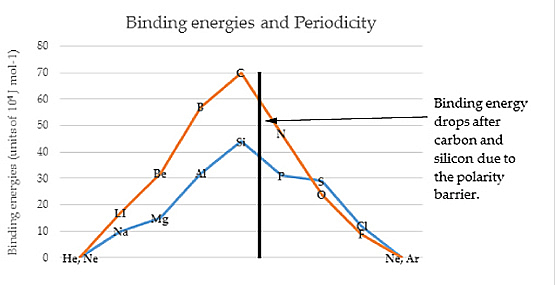
PART VSUB-ATOMIC PARTICLE PHYSICSAlternative Residual Radiation TheoryFor a number of decades a major aspect of cosmology has centered on the Big Bang theory. Isotropic radiation when measured indicate a Kelvin value of about 2.6 degrees. In attempting to account for this residual temperature, a mathematical extrapolition process going backward in time reaching a point of dispersion of matter and energy, now commonly known as the Big Bang. An alternate is suggested. The Cabibbo Angle is evidence of a fundamental inequality between positive and negative segments in both electromagnetic waves and matter itself. The 13 degree factor in scattering experiments strikes at the very core of the Big Bang cosmology.
Planck's constant defines the outer limits of radiation emission and absorption oscillations and it defines the potential difference. This is the source of the residual background radiation. In another area of great interest today, superconductivity, we find that more atoms from one area (oxygen) have to be added to another area (copper) to create the superconducting layers that form in the molecules. How much more? About 6 1/2% to 7% more. |
 |
- Home
- The Circular Model
- Quantum Charts
- Model Physics
- Part I: supporting evidences
- introduction
- Pauli's exclusion principle
- dipole magnet
- unique electron flip
- polarity and anomalous angular momentum
- lanthanide contraction
- Stern-Gerlach
- electron tunneling
- discreteness
- electronegativity
- Compton effect
- Dirac's equation
- symmetry
- gyromagnetic ratio
- nulcear shells
- Kaluza-Klein
- gravity
- magnetism and monopoles
- Heisenburg uncertainty principle
- missing mass
- Olbers' paradox
- Big Bang
- Part II: spectral evidences
- Part III: fine structure constant
- Part IV: superconductivity
- Part V: sub-atomic particle physics
- Part VI: summary
- Part I: supporting evidences
- Astrophysics