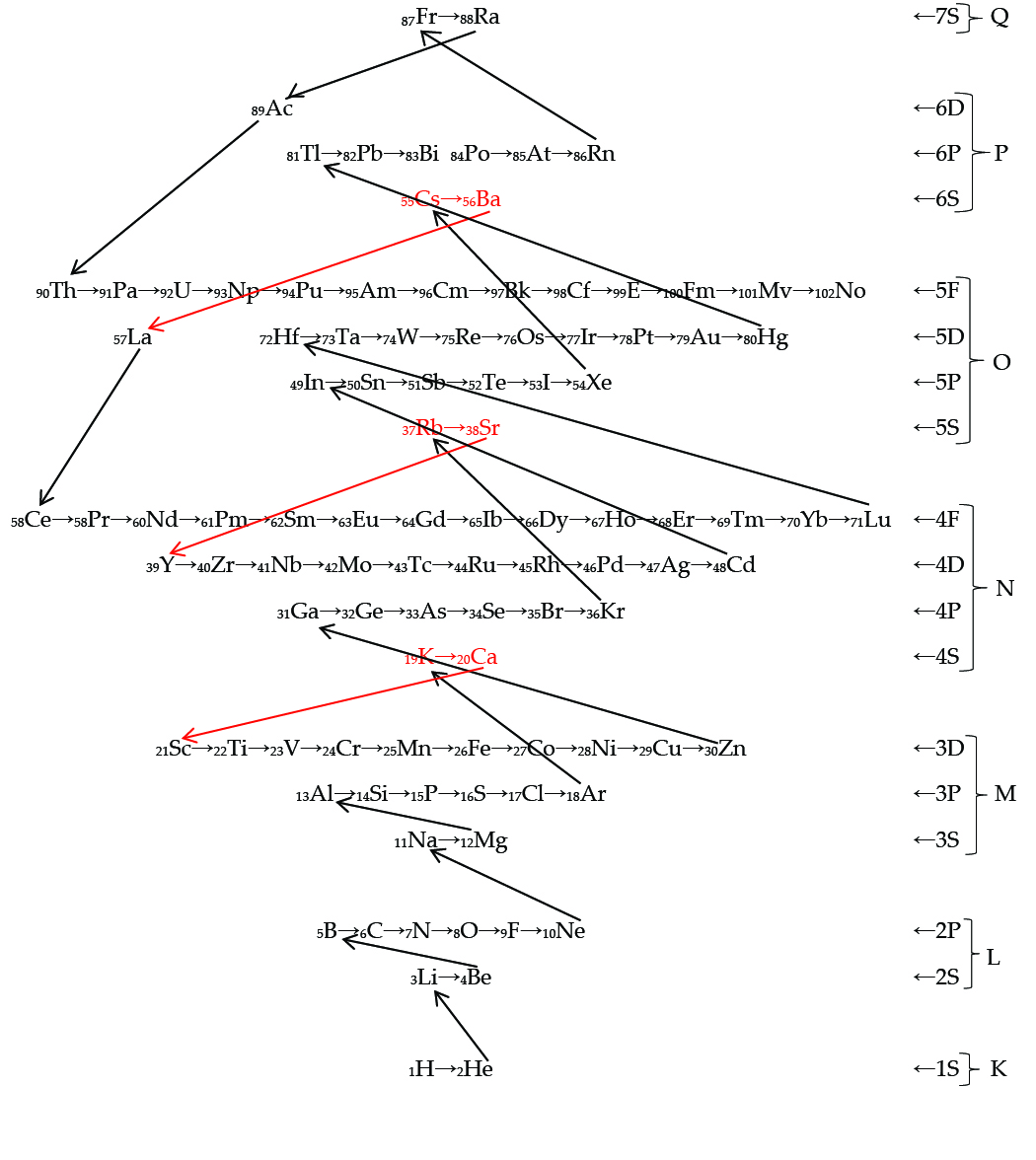
PART IISPECTRAL EVIDENCESDisplaced TermsOne of the unique features of the Circular Model of the Atom is the illustration of the dynamic shifting electron possibilities in the alkali metals and the alkali earth elements. This resulted in spectra of the same element giving rise to different series with slightly shifted lines. The model originators have been chided for illustrating this shifting electron phenomenon in this model. This dynamic shifting electron approach is strongly supported by abundant spectral evidence. In the January 1925 issue of Astrophysical Journal, Henry Norris Russell and Frederick Albert Saunders published a paper devoted to the interpretation of "Many prominent lines in the spectra of calcium and the allied metals which do not belong to the regular series and yet are evidently closely related to them" [1] (emphasis added). By these prominent lines they meant "several very conspicuous groups of lines, of exactly the character now recognized as multiplets, which are evidently combinations between known triple terms and other terms for which no place can be found in the recognized series" [2]. The question arose as to the interpretation of the anomalous terms of calcium. Wentzel attributed it to a 33 orbit , but Lande felt the anomalous term should have the same azimuthal number as the normal series electron i. e. 41. "That is, there existed P-, D-, and F-terms whose energy was shifted with respect to the corresponding normal ones. More accurately, there existed two types of anomalous terms: those which combine with the usual terms having the same azimuthal quantum number and those which combined with usual terms having different azimuthal quantum numbers: the former were denoted as P'-, D'-, and F'-terms and the latter as P''-, D''-, and F''- terms. Anomalous lines occurred rather frequently; for example, of the calcium lines, 81 were normal and 39 were anomalous lines.... Further, it was possible to arrange the new lines in series.... A particularly surprising feature was that some of the anomalous terms, e. g., The 3P'-term in calcium, possessed a negative ionization potential....Nevertheless, these anomalous terms belonged evidently to the neutral and not to the ionized atom" [3]. One of the distinguishing characteristics of the Circular Model of the Atom is the dynamic shifting of electrons in the alkali metals and alkali earth segments of the model. The model demonstrates the phenomena that some elements have "dual personalties or properties." Those elements with that characteristic have been designated "sliders." They occur in the alkali metals and alkali earth groups or octects I and II, and demonstrates the shifting that occurs between the s energy levels to the d energy levels. This may seem unusual at first scrutiny, however, close examination of spectroscopic "displaced terms" confirm this sliding phenomena with elements in the alkali metals and alkali earths. The Circular Model of the Atom provides the structure and demonstrates the shifting of electrons on similar energy levels. Demonstrating as Candler concluded, "This surprisingly large energy is open to only one explanation. In the levels of this displaced series two electrons are excited, and when the atom returns to one of the normal states, both electrons jump and both contribute to a single quantum of radiation" [4]. "Apart from the terms for which only one electron is excited, other terms are possible for which two (or even more) electrons are in shells other than those for the ground state. Such terms are actually observed and are called primed or anomalous terms. They were first observed for the alkaline earths and alkaline-earth-like ions. In their spectra were found multiplets which could not be arranged in the normal triplet series and which did not show the normal structure of a compound triplet” [5]. As the atom fills the various shells, eventually the radius increases until there is more room radially for the electrons to function in an angular momentum shifting manner than in interior shells. What are the causal factors of this shifting? First, a positive dipole in this area acts as a barrier. Second, the law of alternative multiplicities is operational in the filling of the multiplicities which causes atypical shell filling in this area of the periodic table. Third, these are all related to the Madelung effect of shifting energy levels in the d shell. A major feature of the model addresses the irregular filling of energy levels in the alkali metals and alkali earths elements within the periodic table. "For example, 4S-levels (potassium 19, calcium 20) are filled before 3D (scandium 21, zinc 30), or 5S (rubidium 37, strontium 38) before 4D (yttrium 39, cadmium 48), etc. This rule is called Madelung-rule (or Goudsmit rule).... There is to be no complete derivation of the Madelung rule from electronic correlation theory" [6]. The figure below traces the buildup of quantum energy 1 and 2 by atomic number. The elements labeled in red are those above noted elements that fill in an irregular manner.
Citation needed. The Circular Model answers the reasons for the irregular filling of electrons in the alkali earths. These are all accounted for in an integrated manner in the Circular Model of the Atom. [1] Russell, H. N. & Saunders, F. A., 1925. New Regularities in the Spectra of Alkaline Earths. Astrophysical Journal, lxi, p. 38. [2] Russell, H. N. & Saunders, F. A., 1925. New Regularities in the Spectra of Alkaline Earths. Astrophysical Journal, lxi, p. 42 (emphasis added). [3] Mehra, J. & Rechenberg, H., 1982. The Historical Development of Quantum Theory, Vol I, Part 2. New York: Springer-Verlag, p. 688 (emphasis added). [4] Candler, C., 1964. Atomic Spectra and the Vector Model. Princeton: D. Van Nostrand & Co., p. 148 (emphasis added). [5] Herzberg, G., 1944. Atomic Spectra and Atomic Structure. New York: Prentice- Hall, p. 164 (emphasis added). [6] Barut, A. O., 1972. Group Structure and the Periodic System. In: The Structure of Matter, Rutherford Centennial Symposium. Canterbury (New Zealand): University of Canterbury, pp. 126-127. |
 |
- Home
- The Circular Model
- Quantum Charts
- Model Physics
- Part I: supporting evidences
- introduction
- Pauli's exclusion principle
- dipole magnet
- unique electron flip
- polarity and anomalous angular momentum
- lanthanide contraction
- Stern-Gerlach
- electron tunneling
- discreteness
- electronegativity
- Compton effect
- Dirac's equation
- symmetry
- gyromagnetic ratio
- nulcear shells
- Kaluza-Klein
- gravity
- magnetism and monopoles
- Heisenburg uncertainty principle
- missing mass
- Olbers' paradox
- Big Bang
- Part II: spectral evidences
- Part III: fine structure constant
- Part IV: superconductivity
- Part V: sub-atomic particle physics
- Part VI: summary
- Part I: supporting evidences
- Astrophysics