Introduction to theCircular Model of the AtomHelen Pawlowski conceptualized the Circular Model of the Atom after wondering why the standard periodic table looks the way it does. Specifically, she wondered why the lanthanide and actinide series were separated from the rest of the periodic table. In the early 1980s, she looked at electron configuration tables and noticed patterns, or as she called them “spurts” (Pawlowski, 1990, p. 8). Each element had a distinguishing electron that seemed to build as the elements increased in atomic number. She also realized that the patterns seemed to repeat themselves. The following table shows these patterns. The red number identifies the distinguishing electron in the respective electron configurations.
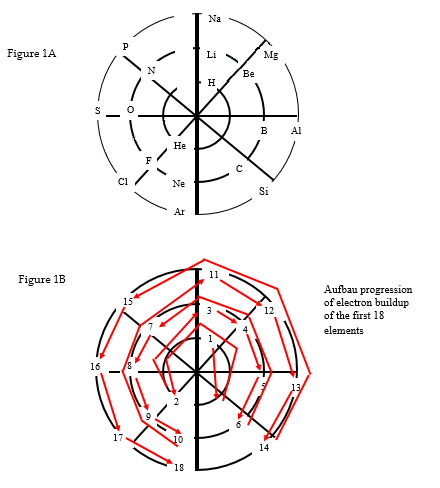
Table adapted from Visualization of the Atom, p. 10. Asterisks denote irregular electron movement. She used the above chart to guide her in building the Circular Atom Model. After identifying the electron buildup, she organized the electron buildup in a modified circular manner; rather than building up in each energy level in a clockwise manner, she reversed the buildup after filling half of the energy level. By reversing order once half the shells are filled, she noticed that the element families maintained their respective orders (i.e., noble gases, halogens, alkali earths, etc. all align in order). The following figures show position of the first elements as well as the placement order.
At the element of copper, there was a logical difficulty. Copper’s distinguishing electron was the same as potassium, and two elements could not be seated in the same position. Moreover their LS configuration of their ground states are the same: 2S1/2. She resolved this problem by moving or “sliding” the first electron to a D orbital position. The mechanism for solving the copper seating was repeated with zinc and calcium, silver and rubidium, cadmium and strontium, gold and cesium, and mercury and barium. Helen noted that these six elements of copper, silver, gold, zinc, cadmium, and mercury are not transitional elements in that their distinguishing electrons are not in the second or third energy level; these elements all had distinguishing electrons in the outermost energy level. This sliding solved two problems: first, how to account for elements with similar electron configurations. Second, it provided a mechanism to account for unusual electron movements that happen regularly between s and d orbital electrons (e.g., between vanadium and chromium, chromium and manganese, nickel and copper, zirconum and niobium, rhodium and palladium, iridium and platinum, and platinum and gold.) The following illustration shows subtle shifts of those electrons coded to the azumuthal S, P, D, F orbitals; for comparison purposes, the electron ionization energies are shown:
In answer to her initial question, the lanthanide and actinide series fit within the spokes of the Circular Atom Model. Helen, at first, conceptualized the model first as a two-dimensional periodic chart. As she worked with the chart, she began seeing it as a way to visualize atomic structure. As electrons build, there is an inherent dipole that creates positive and negative fields, and answers the question of the location of the magenitc structure within the atom. A north/south polarity line created by the electron placement, influenced by electron spins, creates a positive and negative fields identified in the Circular Atom Model.
|
 |
- Home
- The Circular Model
- Quantum Charts
- Model Physics
- Part I: supporting evidences
- introduction
- Pauli's exclusion principle
- dipole magnet
- unique electron flip
- polarity and anomalous angular momentum
- lanthanide contraction
- Stern-Gerlach
- electron tunneling
- discreteness
- electronegativity
- Compton effect
- Dirac's equation
- symmetry
- gyromagnetic ratio
- nulcear shells
- Kaluza-Klein
- gravity
- magnetism and monopoles
- Heisenburg uncertainty principle
- missing mass
- Olbers' paradox
- Big Bang
- Part II: spectral evidences
- Part III: fine structure constant
- Part IV: superconductivity
- Part V: sub-atomic particle physics
- Part VI: summary
- Part I: supporting evidences
- Astrophysics