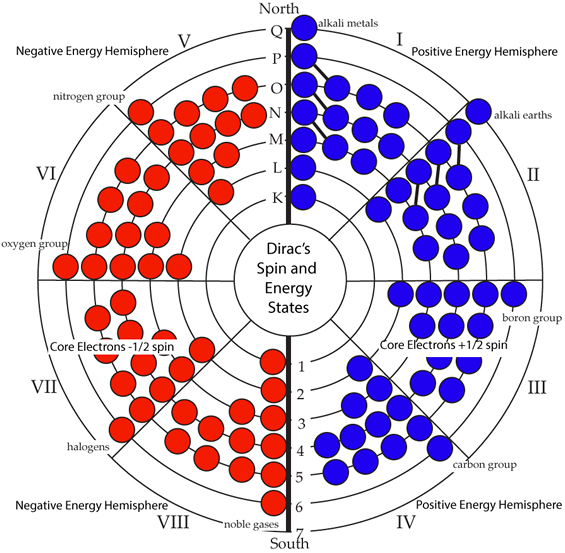
PART IINTRODUCTORY SUPPORTIVE EVIDENCESDirac's EquationDirac's equation of the electron described how the electron can only take on certain specific values and characteristics. “This new equation yields the spin of the electron since it is really a combination of four distinct equations, two of which represent the electron in a state of positive energy and two of which represent the electron in a state of negative energy” [1]. It was the first theory in which the spin of particles was predicted within the equation. His equation predicted that the electron could be in any of four possible states. Spin up, and spin down, were the spin states, and there were two energy states, positive and negative, were derived from his equation. The memorial volume dedicated to Pauli expanded on this concept. “There was one further feature implicit in the wave equation of Dirac which was fully understood only much later. The introduction of a four-component wave function instead of the two component wave function suggested by Pauli entailed a further doubling of the manifold of stationary states: to every state of positive energy there corresponds one with negative energy” [2].
The problem arose from the negative energy states. If they existed, under current concepts, then the atom could not be stable because the electron would drop down into the lower energy state. In his summary section of the Dirac Memorial lecture given by Richard Feynman at Cambridge he stated: “If we insist that particles can only have positive energies, then you cannot avoid propagation outside the light cone. If we look at such propagation from a different frame, the particle is traveling backwards in time: it is an antiparticle. One man's virtual particle is another man's virtual antiparticle” [3]. Adair writes, “[T]he British physicist P. M. Dirac, who first postulated the existence of antimatter, showed that matter and antimatter must behave the same way under electromagnetic forces. Later it was seen that matter and antimatter are acted on identically by gravity and also by the strong nuclear forces” [4]. The Circular Model of the Atom meets the criteria posed by Dirac's four state possibilities of the electron. It has a state for spin up and spin down electrons and they are in a positive and negative field orientation that does not induce annihilation as positron-electron reactions do. The reason there is no annihilation is that each particle in the field has different energy based on ascending levels in the positive field and descending levels in the negative field. [1] Dirac, P. A. M., 1987, The World of Physics. New York: Simon & Schuster, p. 410. [2] Kronig, R., 1960. The Turning Point. In: M. Fierz & V. F. Weisskopf, eds. Theoretical Physics in the Twentieth Century: A Memorial Volume to Wolfgang Pauli. New York: Interscience Publishers, p. 33. [3] Feynman, R. P. & Weinberg, S., 1986. Elementary Particles and the Laws of Physics: The 1986 Dirac Memorial Lectures. Cambridge: Cambridge University Press, p. 55. [4] Adair, R., 1988. A Flaw in a Universal Mirror. Scientific American, February, Volume 258, p. 51. |
 |
- Home
- The Circular Model
- Quantum Charts
- Model Physics
- Part I: supporting evidences
- introduction
- Pauli's exclusion principle
- dipole magnet
- unique electron flip
- polarity and anomalous angular momentum
- lanthanide contraction
- Stern-Gerlach
- electron tunneling
- discreteness
- electronegativity
- Compton effect
- Dirac's equation
- symmetry
- gyromagnetic ratio
- nulcear shells
- Kaluza-Klein
- gravity
- magnetism and monopoles
- Heisenburg uncertainty principle
- missing mass
- Olbers' paradox
- Big Bang
- Part II: spectral evidences
- Part III: fine structure constant
- Part IV: superconductivity
- Part V: sub-atomic particle physics
- Part VI: summary
- Part I: supporting evidences
- Astrophysics