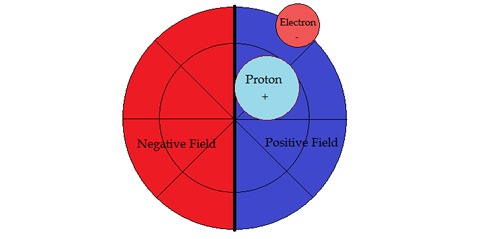
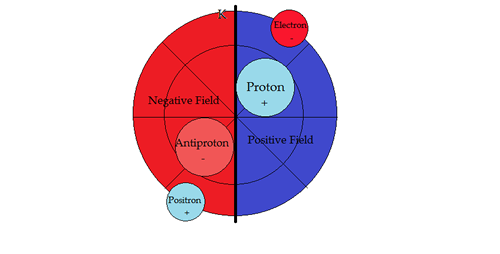
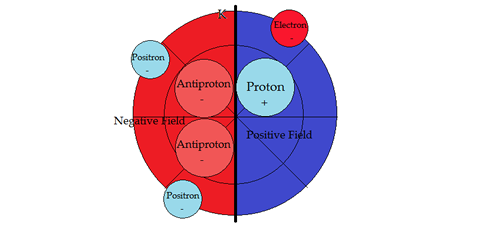
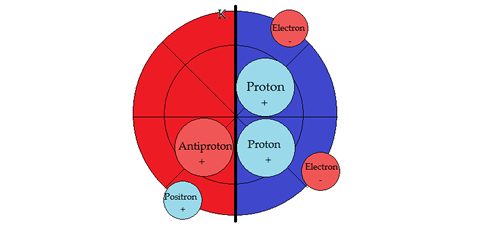
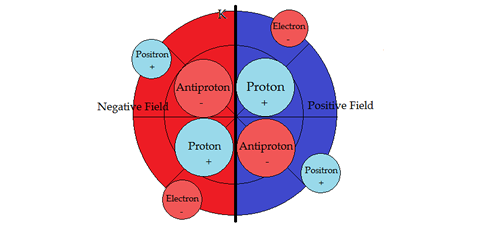
PART VSUB-ATOMIC PARTICLE PHYSICSNucleosynthesisTo help in the descriptions, the vertical line denotes a dipole barrier between positive and negative fields. Second, moving a sub-particle into its opposite field changes its properties to its anti-particle, conforming with the field sign. Hydrogen 1H state
1H Hydrogen: Both proton and electron in positive portion of electromagnetic field. Bohr's theory of energy levels in the atom functions only for `hydrogen like' atoms. Spectral terms of hydrogen emission of Balmer and Bohr's energy levels all occur in Circular Periodic model's positive hemisphere. With more complex structure in heavier elements Bohr and Balmer's energy levels do not work because spectral terms from the negative hemisphere start appearing. The terms originating in negative hemisphere of the atom is the cause of the problem. In the simple hydrogen case the spin states are up, up, for proton and electron. When the spin state of one fermion flips down, it results in a 21 centimeter spectral emission line. This 21 centimeter spectral line is a major tool to astrophysicists. Hydrogen 2H Deuterium state
2H Hydrogen Deuterium is the first compound nucleus, composed of one proton and one neutron. The sign of the proton changes when the particle moves into a negative field. In effect, it is now an antiproton. The characteristics of both leptons and hadrons change not only sign, but attributes as well, upon inversion in movement from positive to negative field or vice versa.
The pair production of sub-atomic particles results in fermions of Feynman while an assistant of John Wheeler's records: “I received a phone call from him in the middle of the night, when he said to me 'I know why all electrons and positrons have the same charge!' Then he explained further, 'They are all the same electron!'....Later on, I was able to make this kind of idea quantitative, by interpreting a positron as being an electron whose phase is going backwards in time" [1]. There is no annihilation because of the substantial mass differential. Annihilation occurs when opposite mass and charge are equivalent. The lack of Deuterium in the universe can be attributed to the type and number of particles being one half stable helium. Hydrogen 3H Tritium state
3H Hydrogen Tritium is the two neutron state of the hydrogen atom. Highly radioactive. the unbalanced neutron decays into proton and beta particles. It doesn't occur naturally, but is artificially produced. HELIUM 3He state
3He Helium has one neutron and two protons whereas the stable isotope of Helium has an additional neutron. The seeming minor addition of one neutron creates entirely different results in Helium particle gun scattering experiments between 3He and 4He. "One finds that the probability of scattering through a 90-degree angle is exactly twice as great for collisions between two 4He particles as for collisions between 3He and 4He, whereas for collisions between two 3He particles, probability of scattering through 90 degrees simply vanishes" [2]. What is the difference between the two helium atoms? The Circular Periodic model has a negative field that changes the scattering amplitudes and probabilities in the Wilczek scattering experiment. The current model does not distinguish boundary conditions between the nucleons. In this case the additional neutron. It is therefore incomplete in providing necessary detailed conditions for nucleosynthesis. In the Circular Periodic model the polarity approach with its negative field provides the structure that distinguishes between 3He and 4He in particle gun scattering experiences. HELIUM 4He state
4He Helium state: Binding energy per nucleon is the highest of all the elements. The nucleon shell structure provides balanced stability in several ways. First, in the positive hemisphere, one (1) proton +, one (1) electron -, then one (1) antiproton - with associated positron +, generating in effect a neutron. Second, in the negative hemisphere, one (1) proton +, one (1) electron - , then one (1) antiproton -, with (1) associated positron +, generating in effect a neutron. This structure is the precursor of the sub-shell closures that occur in a distinct pattern of spin states in both positive and negative hemispheres. The spectral 1S0 states of the alkali earths is evidence of this in the positive field. The ultimate consequence of this arrangement is the effectuation of an antenna type of grouping. (ex. the 2S1/2 & 1S0 terms of the alkali's). This brings the electromagnetic transverse wave components into contact with the particles in the shell structure of the atom. "The fundamental notion of the physics of electromagnetic phenomena is the electromagnetic field. A constant electromagnetic field, independent of time, separates into an electric and magnetic fields. The two are very dissimilar. Nevertheless, the time-dependent electromagnetic field is a unified blend of the electric and magnetic fields. The energy of an electromagnetic wave concentrates alternately in the electric and magnetic fields, in similarity with the potential and kinetic energies of an oscillating pendulum"[3]. [1] Feynman, R. P., 1971. Lectures on Gravitation. s.l.: California Institute of Technology, p. 15-8. [2] Wilczek, F., 1991. Anyons. Scientific American, May, p. 59. [3] Kaganov, M. I. & Tsurkernik, V. M., 1985. The Nature of Magnetism. Moscow: Mir Publishing, p. 8. |
 |
- Home
- The Circular Model
- Quantum Charts
- Model Physics
- Part I: supporting evidences
- introduction
- Pauli's exclusion principle
- dipole magnet
- unique electron flip
- polarity and anomalous angular momentum
- lanthanide contraction
- Stern-Gerlach
- electron tunneling
- discreteness
- electronegativity
- Compton effect
- Dirac's equation
- symmetry
- gyromagnetic ratio
- nulcear shells
- Kaluza-Klein
- gravity
- magnetism and monopoles
- Heisenburg uncertainty principle
- missing mass
- Olbers' paradox
- Big Bang
- Part II: spectral evidences
- Part III: fine structure constant
- Part IV: superconductivity
- Part V: sub-atomic particle physics
- Part VI: summary
- Part I: supporting evidences
- Astrophysics