|
 |
- Home
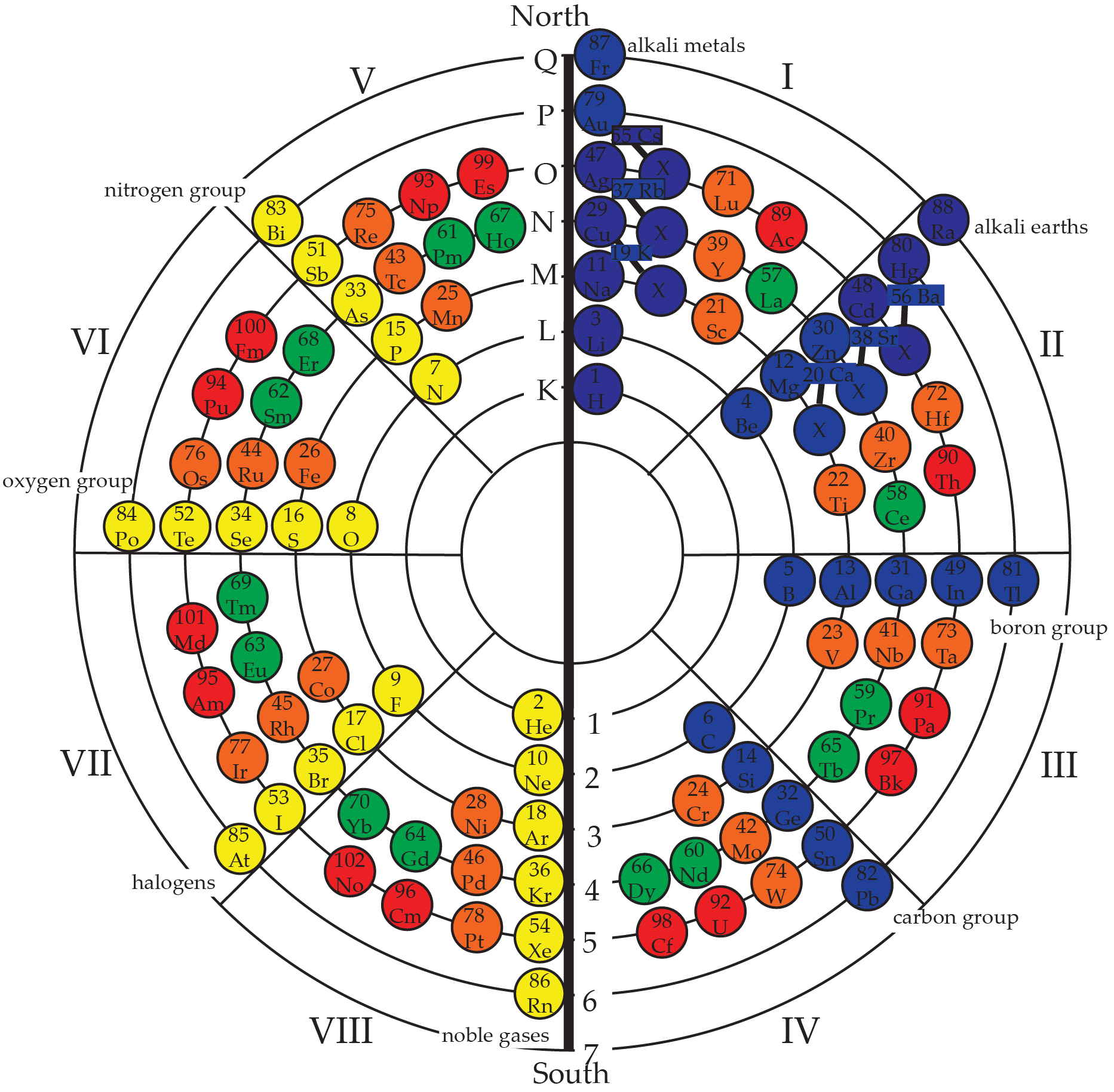
- The Circular Model
- Quantum Charts
- Model Physics
- Part I: supporting evidences
- introduction
- Pauli's exclusion principle
- dipole magnet
- unique electron flip
- polarity and anomalous angular momentum
- lanthanide contraction
- Stern-Gerlach
- electron tunneling
- discreteness
- electronegativity
- Compton effect
- Dirac's equation
- symmetry
- gyromagnetic ratio
- nulcear shells
- Kaluza-Klein
- gravity
- magnetism and monopoles
- Heisenburg uncertainty principle
- missing mass
- Olbers' paradox
- Big Bang
- Part II: spectral evidences
- Part III: fine structure constant
- Part IV: superconductivity
- Part V: sub-atomic particle physics
- Part VI: summary
- Part I: supporting evidences
- Astrophysics